Clusterproject Clean Circles
General
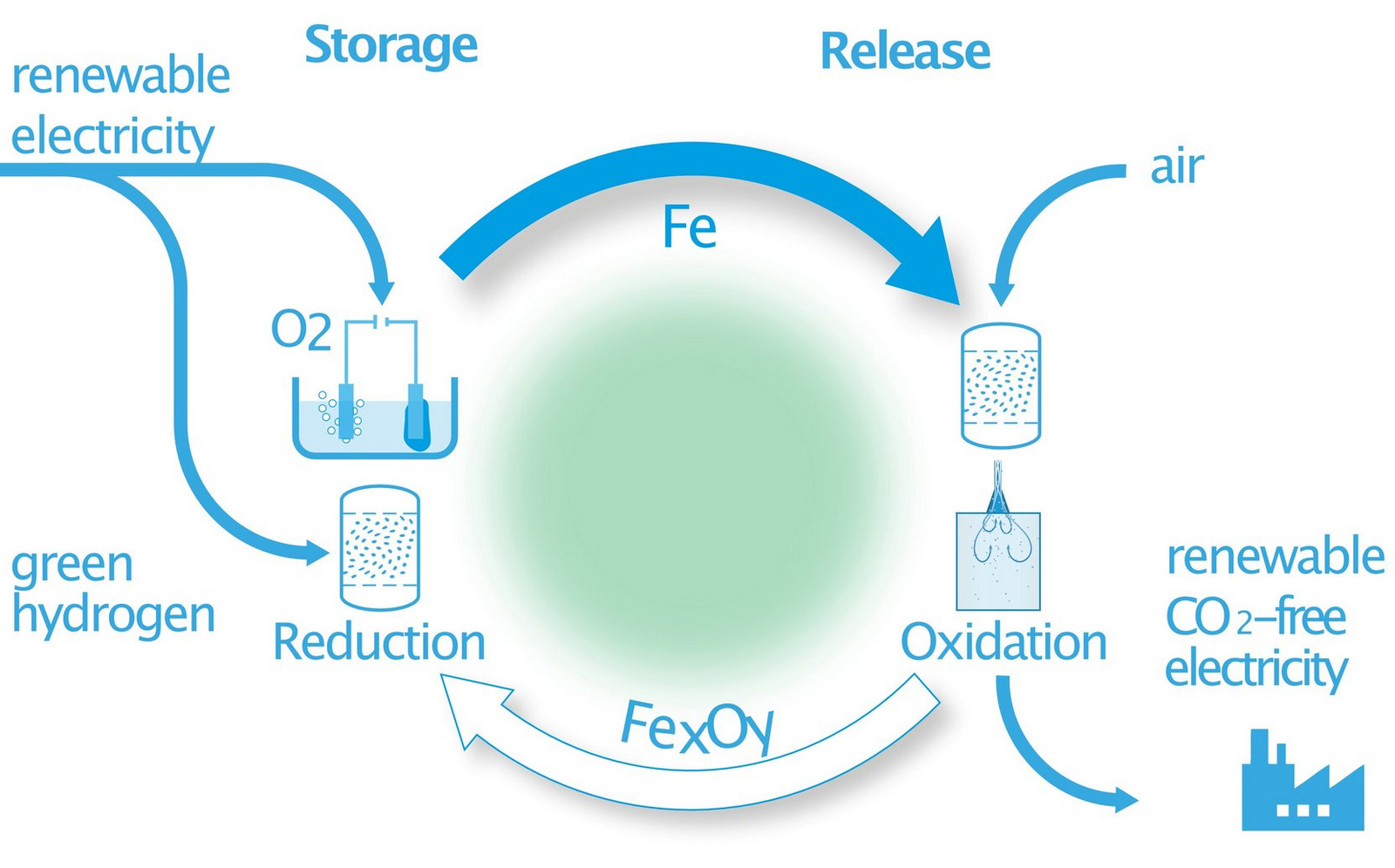
In a unique research approach, iron and its oxides are to be used in a cycle as a carbon-free chemical energy carrier for renewable energy (see figure on the right). Renewable energy is used to reduce iron oxides via a thermochemical or electrochemical process (storage, reduction). Locally and temporally separated from this, elemental iron can be oxidized to release thermal energy for electricity generation (release, oxidation). In this way, renewable energy can be stored in large quantities, transported and made available free of CO2, a hitherto unsolved central challenge of the energy transformation (Energiewende) in the face of changing political conditions.
Iron as an energy carrier has excellent physico-chemical properties with regard to transport, storage and energy utilization. Wind- and sun-rich locations inside and outside Germany can be integrated into a CO2-free circular energy economy for the cost-effective production and usage of renewable electricity. For realizing this environmentally friendly solution, the close and complex coupling of engineering, natural, political and economic science issues must be considered.
For the reduction and oxidation of iron(oxide), chemical (heterogeneous) reactions and their coupling to transport processes must first be understood for isolated processes and individual particles. Reaction rate determining phenomena are heat and mass transfer between particles and the surrounding gas phase as well as the kinetics of the heterogeneous particle surface reaction. These processes are strongly influenced by the ambient conditions (temperature, composition of the gas atmosphere) and the particle properties (particle size, phase, porosity, etc.). Accordingly, a spatially and temporally resolved experimental and numerical analysis of the oxidation and reduction phenomena is required to improve the fundamental understanding.
Working package Prof. Dr.-Ing. Dirk Geyer
None-intrusive, time-resolved diagnostics of iron based micro particles undergoing thermo-chemical reduction or oxidation
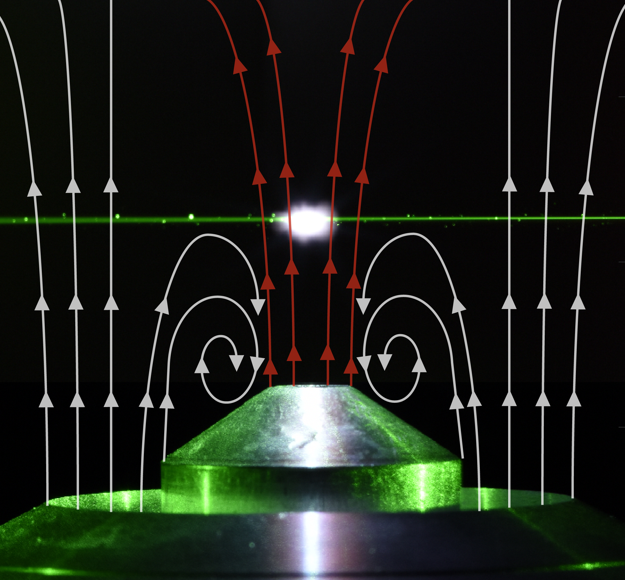
In this subproject, a diagnostic method, enabling a time- and space-resolved in-situ analysis of the elemental composition of iron and iron-oxide particles in flow reactors, will be developed. Due to its high sensitivity, laser-induced plasma spectroscopy (LIBS) will be employed for accessing detailed information on the elemental composition. In LIBS, a pulsed laser beam is focused onto the particle surface and results in absorption of the laser light and the subsequent ablation of a quantity of mass depending for instance on the laser pulse energy or duration. A high temperature plasma is formed around the particle and continuum light is emitted during the early stage while discrete atomic lines are emitted at later times during the relaxation of atoms and ions.
The experimental parameters enabling single-shot experiments for particle diagnostics have to be identified and a data evaluation scheme has to be developed. Quantitative analysis of aerosol particles by LIBS requires an analysis of the relevant physical processes, namely heat and mass transfer during the ablation, which govern the dissociation, vaporization, ionization and ultimately the atomic excitation, of analyte species. These same processes play key roles in particle-related matrix effects, affecting quantitative analysis. Lasers with shorter pulse length have to be found to cause less continuum emission and more pronounced atomic emissions due to reduced electron avalanching and a cooler plasma. Hence, excitation by a picosecond Nd:YAG Laser system will be compared to the commonly employed nanosecond excitation scheme. By now, particles up to 7-10 µm have been fully ionized and subsequently analyzed by LIBS. For larger particles in the range of 30 µm, as relevant in Clean Circles, ionization might be only possible for a fraction of the particle. Hence, the elemental analysis of large particles has to be explored in parametric investigations. A flow configuration which allows for these parametric investigations of single particles will be developed, tested and explored.
Working package Dr.-Ing. Sandra Hartl
Scale-reduced modeling for reduction and oxidation of iron(oxide) considering heterogeneous kinetics and complex mixing processes
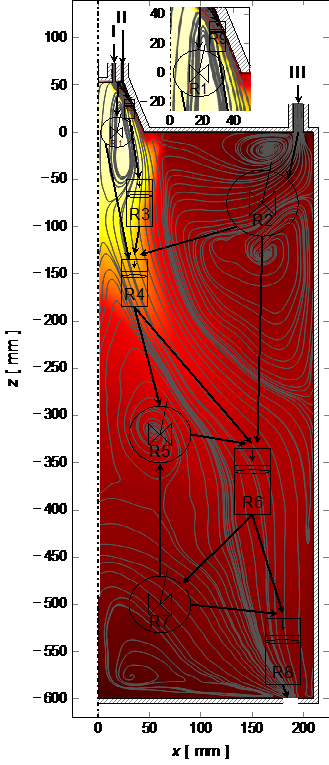
In this subproject, scale- and complexity-reduced approaches (e.g. compartment models, reactor network, zone model, network model) are considered and developed for a comprehensive model, describing reduction and oxidation of iron(oxide). In a compartment model approach, the reactor volume can be subdivided into functional compartments with representative concentrations, temperatures or mixing states. The combination of ideal reactors (flow reactor, perfectly stirred tank) can than, for example, be used to approximate the residence time and turnover behavior of real processes. The coupling of the individual prototype reactor elements takes place via the exchange of, among others, species and energy across compartment boundaries.
The scientific tasks are the development, extension, calibration and application of compartment models to the reduction and oxidation of iron(oxides). Scientific challenges include the extension of reactor network approaches with respect to heterogeneous kinetics of iron(oxides), heating rate effects and the transport of particles across compartment boundaries. Further, a detailed analysis of local and global residence times, sensitivities and validity ranges, with respect to the heat and complex flow, are essential for a consolidation in a comprehensive model. Using CFD results in combination with a (partially) automated division into compartments, an extension of heterogenous kinetics for iron(oxide) and a detailed understanding of local residence times; a new quality of compartment models can be achieved, consolidation in a comprehensive model and applied to technical scale applications, considered within this project.
